The new EU Health Technology Assessment Regulation (HTAR) aims to improve the access of innovative technologies for EU patients. Its goal is to increase the transparency and quality of HTA procedures across the EU, and make efficient use of public resources by providing a centralized and harmonized approach. There are also opportunities for Health Technology Developers (HTDs) stemming from HTAR implementation, including the potential for faster market access of medicines and certain medical devices across the EU in the long term. In addition, the implementation of HTAR may propel HTDs to drive broader organizational enhancements and integration of HTA considerations in product strategy and development.
Despite the aspirational benefits, the published EU HTA implementation acts pose considerable challenges for HTDs such as ambitious timelines and substantial uncertainties on matters like the consolidation approach of Patient/population, Intervention, Comparison and Outcomes (PICO) requirements.
As HTDs prepare for this new regulation, they are adopting various strategies to navigate the transition. By exploring and integrating the latest technological advancements, they could ensure high-quality, efficient EU HTA implementation, turning potential challenges into opportunities for growth and innovation.
Challenges and uncertainties arising from EU HTA
The new EU HTAR will become effective as of January 2025, starting with oncology and advanced therapy medicinal products (ATMPs). Orphan drugs will be subject to the EU HTAR starting from 2028, while all other drugs will follow in 2030. The EU HTA and European Medicines Agency (EMA) regulatory assessments will be run largely in parallel, as shown in Exhibit 1.
Exhibit 1: EU HTA timeline overview
The EU HTA procedure is initiated once the dossier for market authorization (MA) is submitted to EMA. The EU HTA Coordination Group (CG) collects PICO requests from member states, consolidates and communicates those to the HTD, who in turn has a maximum of 100 days to hand-in the EU HTA dossier for the Joint Clinical Assessment (JCA). The JCA outcomes are released after the European Commission (EC) decision on MA, at which point member countries are free to adopt the JCA report or request further evidence.
Leading up to the implementation of the EU HTA regulation, HTDs are facing a number of challenges which will need to be accounted for in their launch and business planning journeys:
How are companies preparing for EU HTA adoption?
Our recent qualitative benchmarking across different HTDs of various sizes, highlighted a number of key strategic considerations being made today to prepare for EU HTA:
- 1EU HTA delivery: How do we deliver high quality HTA JCA dossiers within the defined PICO scope and therefore enable favorable patient access across the EU, in an efficient manner?
- 2Policy shaping: What role do we take in the evolution of the EU HTA regulation implementation (ranging from observing to proactively engaging in discussions on the implementation where possible)?
- 3Governance structure: How do we anchor and further integrate EU HTA capabilities within our organization to optimize JCA outcomes e.g., regulatory and market access, within our organization?
- 4Resource capacity: How do we best allocate and ramp up our resources to deliver the EU HTA requirements to holistically achieve the desired access outcomes in the EU whilst meeting our organization’s strategic objectives?
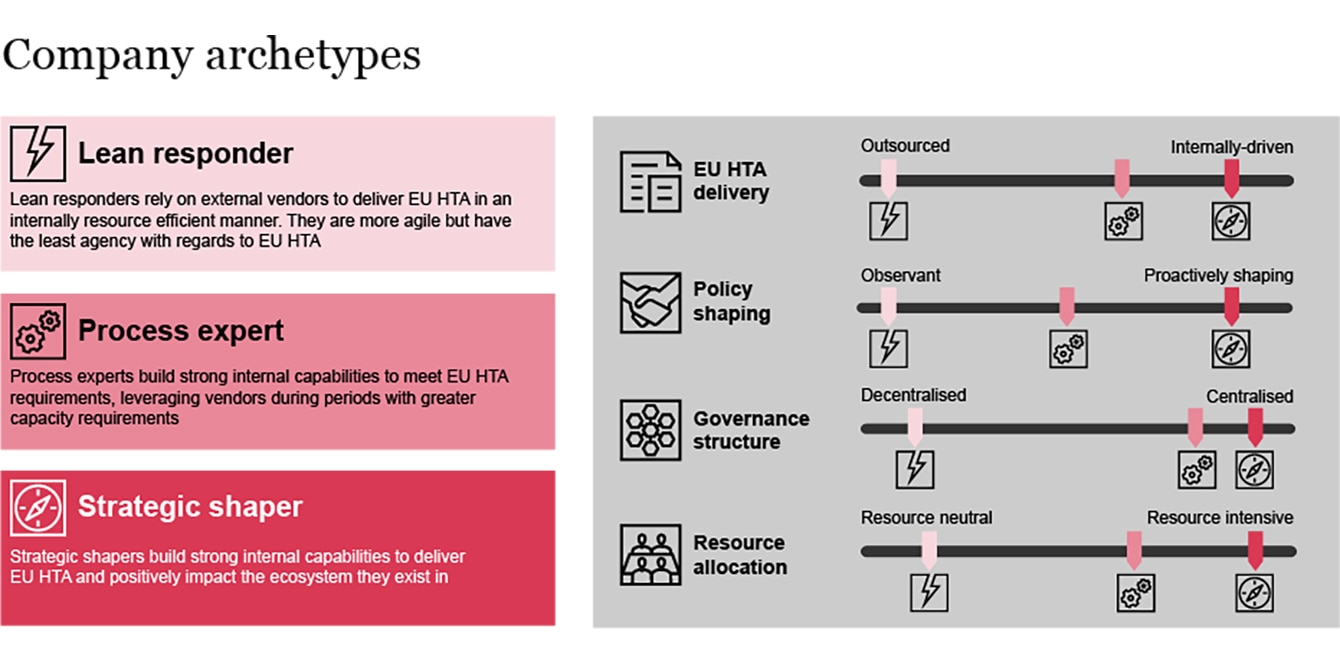
As HTDs prepare for HTAR implementation, based on how companies balance and prioritize the four key strategic considerations, we have observed three distinct archetypes arising:
Exhibit 2: EU HTA implementation company archetypes
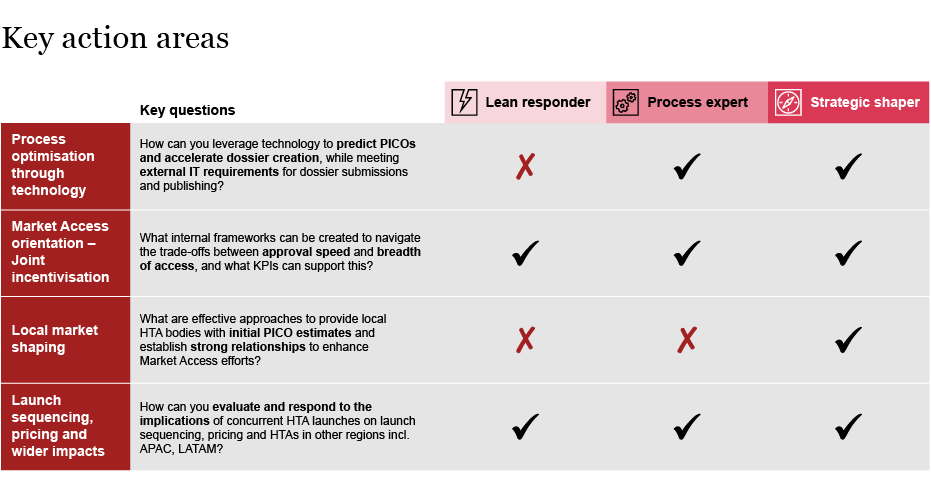
In general, there is no ‘gold standard’ approach; all archetypes serve a given purpose at a specific point in time, provided they are well executed. Whilst HTDs need to make a strategic choice which archetype to pursue initially, they may evolve into another archetype as circumstances and strategies change. The pursuit of a given archetype needs to take into account the company's strategic direction, positioning and pipeline. Once this decision is made, the execution of each archetype is determined by a number of key success factors:
Lean responders
- Robust external delivery network: establishing a streamlined process for selecting and managing an external delivery network, incorporating country-specific considerations and ensuring vendor accountability through a transparent system
- Continued market surveillance: regular reviews and continuous market surveillance are essential to adapt to regulatory changes and competitor actions, ensuring the delivery network meets evolving EU HTA needs
Process experts
- Culture of continuous learning: to achieve process excellence, the organization must cultivate a culture of continuous learning if it does not do this already, regularly reviewing and adapting to changes
- Strong internal communication channels: keeping teams informed and preventing knowledge gaps
- Access to external vendors if needed: maintaining relationships and collaborating regularly with external vendors for additional support during peak times
Strategic shapers
- Culture of continuous learning: actively building expertise and continuously identifying areas for improvement to achieve process excellence
- Effective stakeholder engagement: building strong relationships with HTA bodies, pharmaceutical associations, and patient and clinical experts in order to shape EU access-related procedures and initiatives
- Consistent messaging and actions: alignment across countries on external-facing activities to enhance the receptiveness of regulatory bodies to shaping efforts
Although there are benefits and drawbacks associated with each archetype, they all present opportunities to derive value through effective execution. HTDs that strategically choose the archetype that most aligns with their organizational direction and deliver against the key success factors, will strengthen their competitive advantage. Furthermore, HTDs can pivot their direction if their strategic priorities change. For example, HTDs starting as a ‘lean responder’ might mature towards being a ‘process expert’ or ‘strategic shaper’. Throughout their implementation journey, HTDs should regularly and intentionally re-evaluate their approach, as their pipeline and commercial needs evolve.
Must-haves for successful HTAR implementation
During our benchmarking exercise, it became apparent that HTDs are already undertaking a number of “no regret moves” to prepare for the HTAR implementation:
- Build understanding of the new EU HTAR (EU/national/overall) including regulatory bodies, procedure, pathways, payer needs and its impact on evidence generation
- Foster closer cross-functional collaboration, especially between market access and regulatory functions to align processes, avoid duplication of effort and ensure high quality dossiers are ready at submission
- Ensure importance of EU HTA is communicated and understood across the organization to drive strong portfolio-level accountability and priorities across assets and teams
- Establish local readiness to embrace the new EU HTA including upskilling country teams on the JCA procedure, timelines, as well as gaining their input on critical topics for the development of the dossier
Key future implementation enablers
Beyond the above steps, companies need to think through how to enable their organization to more effectively adapt to HTA changes and capture the opportunities. We have identified four central considerations illustrated below and we are exploring these in more detail.
Exhibit 3: Enablers to successfully adopt EU HTA
Example: Adopting and benefitting from the latest technology to support EU HTA
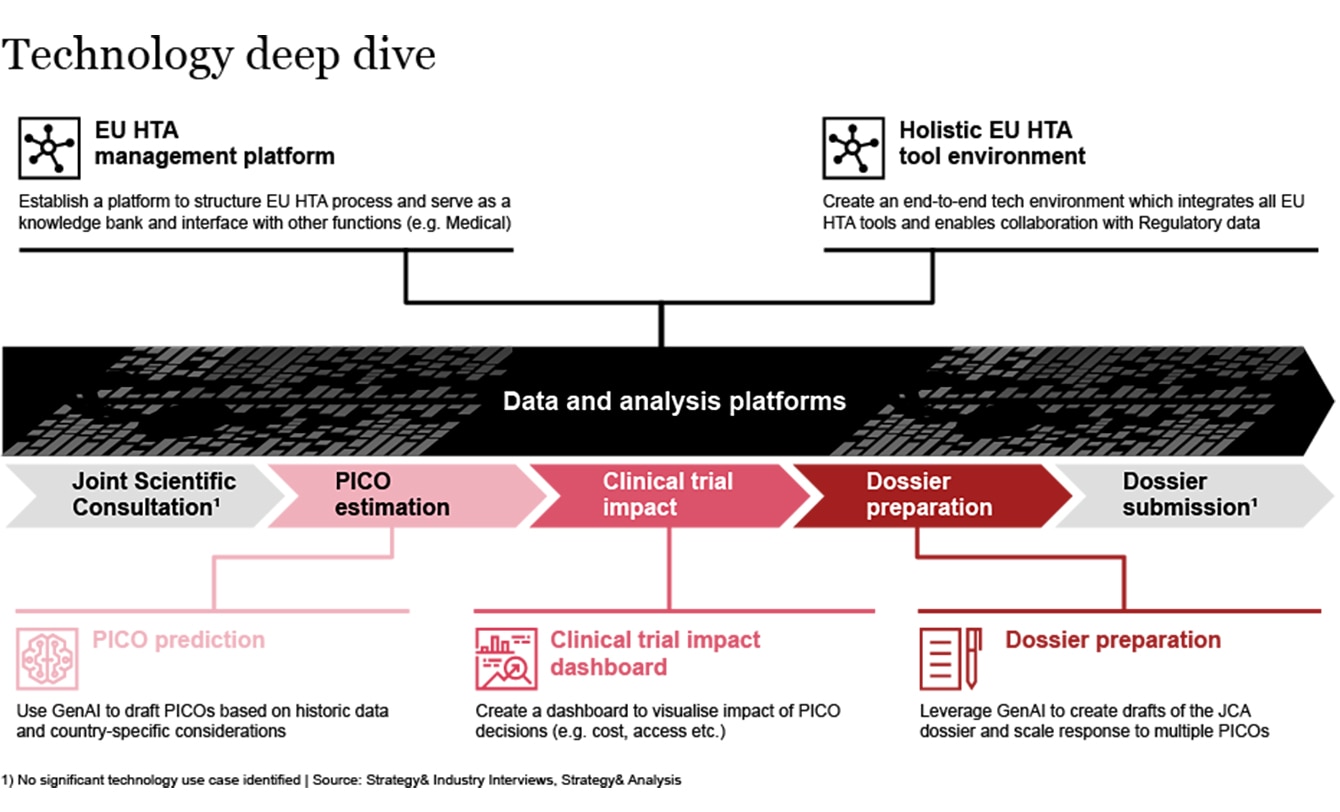
Whether planning for one or more submissions, HTDs will likely grapple with resourcing challenges to meet the tight deadlines and prepare comprehensive dossiers which answer the PICO scope. Technological solutions may alleviate some of the pressures faced by HTDs when delivering on EU HTAR. Leveraging latest technologies, like GenAI, has the potential to streamline and optimize processes, freeing up capacity for teams to focus on driving value that can only be unlocked by human ingenuity and creativity.
For example, in order to meet the 100 day dossier turnaround timeline, it is imperative that HTDs engage in significant frontloading either through PICO prediction before clinical trials; measuring the impact of PICO decisions; or indeed preparing the dossier (scenarios) for JCA submission.
Exhibit 4: Technological solutions as enablers of EU HTA adoption
It is important to acknowledge that access to technological advancements is available to all stakeholders in the ecosystem and a future where the member state coordination group on HTA is also leveraging GenAI, for example to conduct their assessments, may not be too distant. This sparks additional questions like what are the potential risks and opportunities that may arise from all stakeholders in the ecosystem using GenAI.
Call to action for PharmaCos
The new EU HTA regulation presents both challenges and opportunities for HTDs. As the industry navigates the implementation of the novel regulation, HTDs may need to adopt strategic approaches to enable them to materialize the potential for faster market access and drive their own organizational enhancements. It is imperative for HTDs to stay informed, especially in the first few months of implementation, learn from the first JCA outcomes, collaborate cross-functionally and enable the organization to adapt at speed following any updates to the annual work program set by the Coordination Group. Depending on their company archetype, some HTDs may also wish to engage proactively with regulatory bodies to optimize outcomes. The call to action for HTDs is to embrace the changes brought by the EU HTAR, prepare comprehensively for the new requirements, and turn potential challenges into opportunities for growth and innovation. By doing so, HTDs can ensure high-quality, efficient EU HTA delivery and maintain a competitive edge in the evolving healthcare landscape.
Khushbu Goulden, Sebastian Schwärzler, João Aguiar Machado, Elina Osoianu, Lukas Rabl and Armaan Bajaj also contributed to this report.