Medical Affairs has always played a crucial role throughout a product’s lifecycle. However, the function has never previously been so well positioned to lead enterprise strategy and generate both internal and external change.
Due to recent changes in the healthcare and pharmaceuticals industries, Medical Affairs (MA) can take advantage of its existing and evolving capabilities to broaden its scope, and not limit itself to providing information to healthcare professionals (HCPs) through medical education. Because the function is involved across the pharma value chain, from early scientific leadership pre-launch to external stakeholder engagement post-launch, MA professionals have the opportunity to lead strategic decision making. Moreover, crucial MA capabilities, such as medical insights and real-world evidence generation, create unique opportunities to shape enterprise strategy.
There are several factors driving this transformation
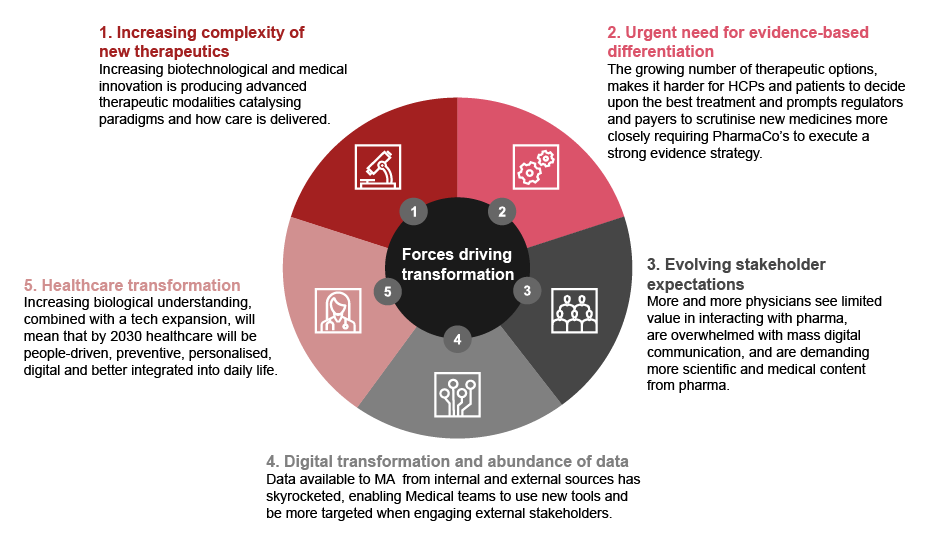
- 1Increasing complexity of new therapeutics
The accelerating pace of biotechnological and medical innovation is producing advanced therapeutic modalities, such as cell and gene therapies. As a result, more complex modes of action, applications and suitability across patient types need to be explained to all stakeholders - HCPs, patients and payers alike. In addition, new therapies are catalyzing new treatment paradigms and how care is delivered.
MA implication: Full-scale adoption requires coordinated, cross-functional efforts. Medical Affairs is a logical choice to connect these efforts in an organization. - 2Urgent need for evidence-based differentiation
The growing number of therapeutic options makes it harder for HCPs and patients to decide upon the best treatment and prompts regulators and payers to scrutinize new medicines more closely. This requires pharmaceutical companies to execute a strong evidence strategy to suit the needs of different healthcare stakeholders. Moreover, there is a need for more innovative evidence types and novel ways of evidence generation, such as collaborative research models or innovative clinical trial designs.
MA Implication: By working closely with other partners such as Clinical Development and Translational Medicine, Medical Affairs is poised to take the lead role in these areas. - 3Evolving stakeholder expectations
According to a 2020 Strategy& physician survey in Germany, only 28 percent of the doctors cite long-standing relationships with the pharmaceutical industry as a critical differentiating factor, thus undermining one of the main pillars of the traditional go-to-market model. Furthermore, an increasing number of physicians are overwhelmed with mass digital communication, and are demanding more scientific and medical content from the industry. Growing constraints on sales teams’ access to HCPs and other stakeholders are also compelling the industry to rethink its engagement and focus on high-quality medical content. In addition, a more interconnected stakeholder environment is weakening the decision-making power of individuals, while opening up improved opportunities for networks, such as physician societies and patient groups.
MA Implication: These trends should make MA representatives aware that they are in an ideal position to take a leading role in the new era of stakeholder engagement. - 4Digital transformation and abundance of data
Data available to MA from internal and external sources has skyrocketed, enabling teams to use new tools and be more targeted when engaging external stakeholders. Real-time analysis of data, for example from claims, IoT devices, electronic medical records (EMRs), external publications, internal research, real-world evidence and other sources, allows activities to be refined for the greatest impact, and in more visible ways than before.
Aside from more efficient data loops, the greater use of new formats, such as digital platforms, can improve stakeholder engagement. New digital avenues, such as the growth of social media, have led to the emergence of medical influencers, who interact with relevant stakeholders in new ways.
MA Implication: These trends should make MA representatives aware that they are in an ideal position to take a leading role in the new era of stakeholder engagement. - 5Healthcare transformation
The way we think about, manage and experience health is fundamentally changing. As outlined in our “Future of Health” (2021) and “From healthcare to life care” (2023) studies, increasing biological understanding, combined with a growing number of viable technology platforms and other trends, will lead to a healthcare environment that by 2030 will be people-driven, preventive, personalized, digital and more deeply integrated into daily life.
MA Implication: New regulatory, organizational and business models will facilitate this evolution, and Medical Affairs is positioned to steer pharmaceutical companies into this new era of healthcare.
All these trends are changing the industry. Now is the time for Medical Affairs to seize the opportunity to guide pharmaceutical companies’ strategic path. Early adopters have already embarked on this journey, optimizing the capabilities of a future-proof Medical Affairs organization.
Brian Selvarajah, Dr. Christoph Gross, Eleanor Craggs and Kevin Kalinka also contributed to this report.
Contact us


